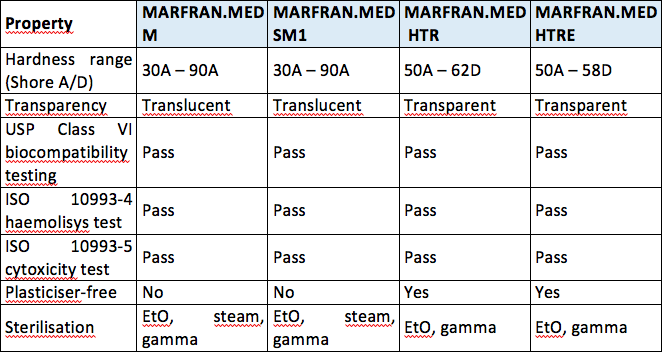
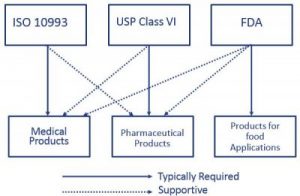
usp class vi vs iso 10993
By ensuring that a material is non-toxic and wont result in immunological rejection biocompatibility testing ensures that a rubber is safe for use with. All the TPUs supplied by ICP DAS BMP are USP Class VI certified and compliant with the ISO 10993 international standard.
Usp31nf26s1 C1031 General Chapters 1031 The Biocompatibility Of Materials Used In Drug Containers Medical Devices And Implants
Evaluation and Testing recommendations outlined in.

. For safety and sterility multiple materials may have to mate virtually seamlessly and this is an area in which overmolding excels. Per FDAs Blue Book Memorandum G95-1 Use of International Standard ISO 10993 Biological Evaluation of Medical Devices Part 1. USP Class VI ISO 10993 and biocompatibility.
Materials have passed skin sensitization and cytotoxicity tests according to ISO 10993-5 and ISO 10993-10. Both ISO 10993 and USP Class VI define testing requirements for biocompatibility the ability of a material to perform a desired function without causing adverse effects on the human body. ICP DAS BMP has its own laboratories for polymerization physical properties analysis and cytotoxicity testing.
There are many other reasons to use overmolding including. In many cases no single resin can meet all of the requirements.

Medical Grade Cyanoacrylate Super Glue Iso 10993 And Usp Class Vi

Regulatory Guidelines For Biocompatibility Safety Testing Mddionline Com
Brilliant Mind The World Of Tubing For Medical Use Medical Plastics News

Understanding Food Grade Vs Biocompatibility For Medical Device Materials Medical Product Outsourcing

Usp Class Vi Certification Presco Marking Products And Engineered Films
Material Selection Medical Injection Molding Xcentric Mold

Usp Class Vi Foster Corporation
Usp31nf26s1 C1031 General Chapters 1031 The Biocompatibility Of Materials Used In Drug Containers Medical Devices And Implants

Iso 10993 Vs Usp Class Vi Medical Molding And Bicompatible Rubber The Rubber Group

Understanding Food Grade Vs Biocompatibility For Medical Device Materials Medical Product Outsourcing

Pre Colored Medical Abs Compounds For Laser Marking Plastics Technology
Usp31nf26s1 C1031 General Chapters 1031 The Biocompatibility Of Materials Used In Drug Containers Medical Devices And Implants

Regulatory Guidelines For Biocompatibility Safety Testing Mddionline Com

Regulatory Guidelines For Biocompatibility Safety Testing Mddionline Com



